R&D
Diagnostic Reagent Laboratory

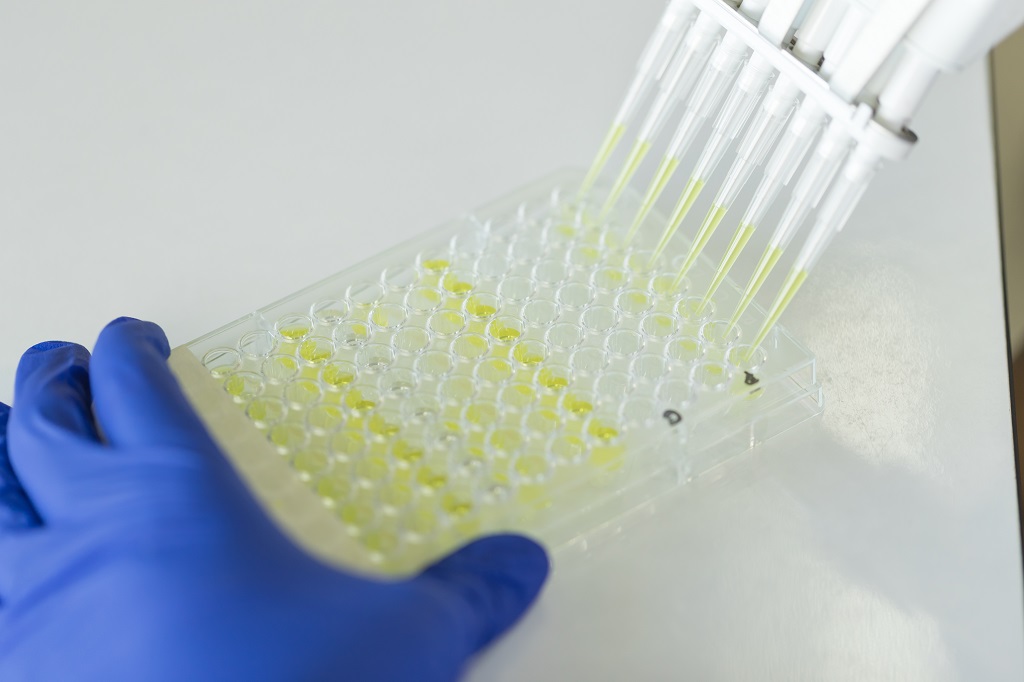
The In-vitro Diagnostic Medical Devices Laboratory of Shinjin Medics Inc. was founded in 1999 on the idea of "Becoming a world leader in diagnostic reagent-realated field".
From the beginning, it has performed a variety of research activities and possessed Hepatitis, Tumor marker, Triple marker, Infectious disease, Hormone and the like. It has brought satisfactory results by stretching list of products and improving performance as a result of continuous studies.
R&D Achievement
First, it developed our own RACER method to maximize the efficiency of our products. RACER is a technology that can produce most test results in less than an hour, and for the first time in the industry, it reduced the testing time of tumor marker tests such as CA19-9 IRMA to less than an hour.
Second, it developed and commercialized the Ultra-sensitive HBsAg, and the Ultra-sensitive PSA as the first product of Radioimmunoassay. We succeeded indeveloping the 3rd generation TSH IRMA product that can detect up to 0.005 ng/ml.
R&D History
- 1999
- Founded Laboratory
- 2000
- Developed RIAKEY Anti-HBs, HBsAg
- 2001
- Developed RIAKEY PSA, CA15-3, CA19-9, CA125
- 2002
- Developed DIAKEY Ant-HBs, HBsAg, HIV1/2
- Developed RIAKEY TSH, and other 4 items
- 2004
- Developed RIAKEY Anti-HBc IgG
- 2005
- Developed DIAKEY H.Pylori IgG, AFP
- 2006
- Developed "RACER Bio Nano Technology" Method
- Developed DIAKEY Free Estriol, Triple Intact HCG, RIAKEY Anti-HBc IgM
- 2007
- Developed DREAM GAMMA-10 and Approved from GMP
- Developed DIAKEY CEA, CA19-9, PSA and RIAKEY T3, T4
- 2008
- Founded Diagnostic Instrument R&D Laboratories
- Developed TW-300+, SI-600, DS Series
- Developed RIAKEY Anti-TPO, TgAb, FT4, Ferritin, NSE
- 2009
- Developed RIAKEY TG
- 2010
- Developed RIAKEY Ultra-sensitive PSA and other 12 items
- 2011
- Developed RIAKEY Monototal and other 4 items
- 2012
- Developed DIAKEY Rapid HBsAg, H.Pylori
- 2013
- Developed DIAKEY Rapid DOA6, Syphilis and other 12 items
- 2014
- Developed
- 2016
- Developed MASSIA E-2600 (Fully Automated Instrument for ELISA)
- 2017
- Developed MASSIA R-4200 (Fully Automated Instrument for RIA)
Diagnostic Instrument Laboratory
Diagnostic Instrument R&D Laboratories at Shin Jin Medics Inc. concentrates its efforts to develop robotic system and software for diagnostic medical device automation by combining Bio with IT. It has continued to work on domestic medical device automation which lagged behind so far and developed , beginning what GAMMA-10, one of DREAM (Diagnostic Reagent Enhanced Automatic Mechanism) series, such as TW-300+, SI-600 and other automatic instruments. Following products are developed successively; RALS (Radioactive Automatic Laboratory System) which is RIA Laboratories Automation System and software specialized in Life Science like FETUSCREEN which is designed to test for congenital malformation by Quadruple and Triple Marker.
We, Shin Jin Diagnostic Instrument R&D Lab., will keep our vocation regarding human healthcare and welfare and be the finest by establishing and researching new mechanisms.


